
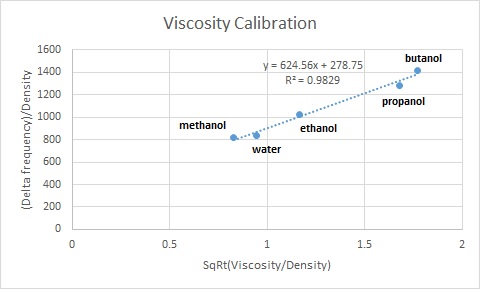
Viscosity is a measure of the resistance of a liquid to flow. Liquids like acetone and water have a low viscosity, since there is little resistance for the molecules to overcome. Substances like molten glass and pitch, on the other hand, have very high viscosities and flow very sluggishly. Some liquids have viscosities that vary with shear rate – the classic example is cornstarch and water (oobleck), which acts more like a liquid when it’s strained slowly, and like a solid when you strain it quickly (which is why you can run across a pool of it). In this lab, we do a calibration of the Quartz Crystal Microbalance (QCM) with liquids of known viscosity, and then use that calibration to calculate the viscosity for unknown mixtures of various concentrations (water and alcohol, and of two alcohols mixed).
Equipment Used
- Quartz Crystal Microbalance (QCM)
- Quartz crystals with plated electrodes
- Analytical balance
- Graduated cylinders
- Disposable pipettes
- Brookfield Rotational Viscometer
Materials Tested
- Liquids of known viscosity: methanol, ethanol, propanol, butanol, and water
- Mixtures of alcohol plus water, and two different alcohols
- Poly(ethylene oxide) in acetonitrile solution (for Brookfield demo)